Abstract
Background: The pattern of multiple myeloma (MM) relapses includes both biochemical relapses, characterized by the increase of monoclonal paraprotein in absence of organ dysfunction and CRAB symptoms, and clinical relapses, when the increase of monoclonal protein is associated with organ dysfunction. The International Myeloma Working Group (IMWG) recommends starting the subsequent treatment when either clinical relapse or a significant paraprotein increase occur (Rajkumar S. V. et al., Blood 2011 Feb 3; 1st ed.). To date, no data are available about the benefit of treatment at biochemical relapse rather than waiting for clinical relapse. Data from the RETRIEVE study suggested that re-treatment with bortezomib is safe and effective (Petrucci M. T. et al., Haematologica 2010; 95 (s2):152). Here we report the results of a phase II multi-center, randomized study that investigated the efficacy and safety of bortezomib re-treatment at biochemical relapse compared to observation until clinical relapse.
Methods: MM patients, relapsed and/or refractory (RR) after 1 to 3 prior lines of anti-myeloma treatment, who received salvage bortezomib-based treatment as last-line therapy without evidence of disease progression, were eligible for enrolment in the protocol. Patients who had at least a stable disease to previous salvage bortezomib-based treatment could be enrolled within 45 days from the completion of the previous salvage cycle. Patients were randomized (1:1) to either receive treatment at the occurrence of biochemical relapse with six 4-week cycles of subcutaneous (SC) bortezomib (1.3 mg/m2 on days 1, 8, 15, 22) plus dexamethasone (40 mg on days 1,8,15,22) (Vd, arm A) or no treatment being administered until clinical relapse (arm B).
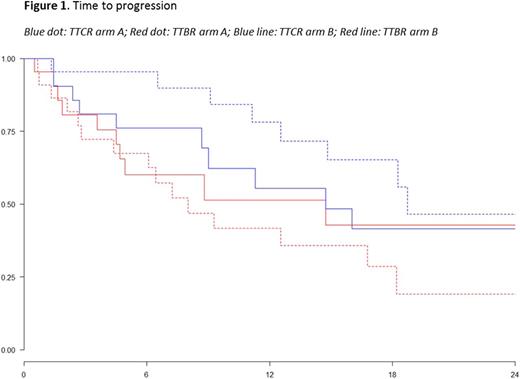
The primary endpoint of the study was time to progression (TTP), calculated from the time of enrolment to both biochemical relapse (TTBR) and clinical relapse (TTCR). Secondary endpoints included: progression-free survival (PFS), overall survival (OS), and overall response rate (ORR). Responses and progressions were assessed by investigators according to IMWG criteria. Time-to-event endpoints were estimated by using the Kaplan-Meier method.
Results: A total of 46 patients were enrolled in the two arms (arm A=23; arm B=23). Median age was 72 (63-83) and 69 years (48-85; p=0.02), and ISS stage II/III was 35% and 26% (p=0.75) in arms A and B, respectively. The median number of prior therapies was 1. At enrolment, the ORR to prior bortezomib-based salvage treatment was 91% and 83% in arms A and B, respectively.
After a median follow-up of 15.7 months, 65% (15/23) of patients in arm A and 48% (11/23) of patients in arm B had a biochemical relapse. All 15 patients in arm A with a biochemical relapse started re-treatment with Vd (median number of cycles administered: 4; range 1-6). Among evaluable patients (n=12), best responses to re-treatment were: very good partial response (VGPR) in 17% of patients (2/12), partial response (PR) in 33% (4/12 patients), stable disease (SD) in 17% (2/12), and progressive disease (PD) in 33% (4/12). In arm B, 9/11 patients in biochemical relapse had a subsequent clinical relapse while 2/11 remained asymptomatic. One-year TTBR was 42% vs 51% (HR 1.22, p=0.61) and 1-year TTCR was 78% vs 55% (HR 0.54, p=0.19) in arm A and arm B, respectively (Figure 1). One-year PFS2 was 84% in arm A and 71% in arm B, respectively. No significant differences were observed in the rates of any grade haematological (p=ns) and non-haematological (p=ns) adverse events (AEs) between the two arms. Among patients in arm A, neither grade 3-4 nor severe treatment-related AEs were reported. Treatment-related grade 1-2 neutropenia, anemia and thrombocytopenia were observed in 9%, 4%, and 4% of patients, respectively. Peripheral neuropathy (13%) and hypertension (9%) were the most frequent grade 1-2 treatment-related non-haematological AEs.
Conclusions: in myeloma patientstreated at relapse with a bortezomib-based regimen, early re-treatment with bortezomib at biochemical relapse was feasible and effective in delaying clinical progression as compared to observation alone. Further follow-up is needed to assess long-term outcomes. Updated data will be presented at the meeting.
Larocca: Janssen: Honoraria; Celgene: Honoraria; Bristol-Myers Squibb: Honoraria; Amgen: Honoraria. Offidani: celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cellini: Celgene: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy. Caravita di Toritto: Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Travel grant; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grant; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grant; Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Petrucci: Celgene: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Amgen: Honoraria. Musto: Janssen: Honoraria; Celgene: Honoraria. Palumbo: Genmab A/S: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Binding Site: Research Funding; Takeda: Consultancy, Employment, Equity Ownership, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Sonneveld: Celgene Corporation, Amgen, Janssen, Karyopharm, PharmaMar, SkylineDx: Honoraria; Celgene Corporation, Amgen, Janssen, Karyopharm, SkylineDx, PharmaMar: Consultancy; Celgene, Amgen, Janssen, Karyopharm, Takeda: Consultancy, Honoraria, Research Funding. Boccadoro: Mundipharma: Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Abbvie: Honoraria; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal